Sugar
The structure of the APSIM sugar model can be described as follows:
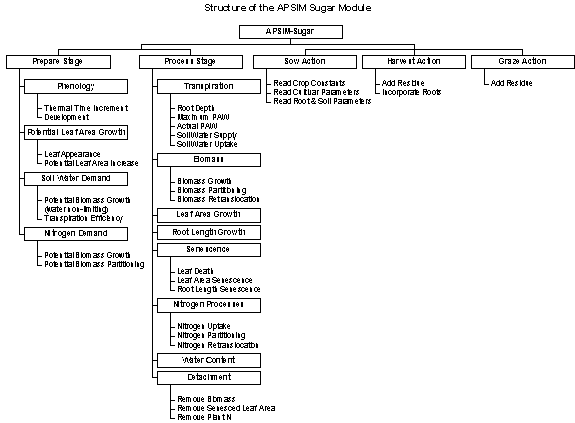
Order of Calculations
For each APSIM time step the calculation of sugar model states and transitions are performed in a set order in different stages of the APSIM cycle through each time step.
Phenology and potential growth and demands are calculated during the prepare stage and actual growth and changes to state variables are calculated during the process stage.
Parameterisation
Structure of the INI file
There are five separate categories of variables in the sugar module’s ini file.
They are listed below with some examples of the type of parameters included in each.
Constants
Upper and lower bounds for met and soil variables
Plant_crop
Growth and partitioning parameters
Water Use Parameters and Water and temperature Stress Factors
Frosting Factors
Nitrogen Contents and Nitrogen Stress Factors
Ratoon_crop
Same as Plant crop section but there is the ability to change the parameters between plant and ratoon crops.
Cultivar Plant Crop
Leaf Development Parameters
Phenology
Sucrose and cane stalk Partitioning Parameters
Cultivar Ratoon Crop
Same as Plant crop section but there is the ability to change the parameters between plant and ratoon crops.
Model Components
Overview
Crop dry weight accumulation is driven by the conversion of intercepted radiation to biomass, via a radiation-use efficiency (RUE).
RUE is reduced whenever extremes of temperature, soil water shortage or excess, or plant nitrogen deficit limit photosynthesis.
The crop leaf canopy, which intercepts radiation, expands its area as a function of temperature, and can also be limited by extremes of temperature, soil water shortage or excess, or plant nitrogen deficit.
Biomass is partitioned among the various plant components (leaf, cabbage, structural stem, roots and sucrose) as determined by crop phenological stage.
Nitrogen uptake is simulated, as is the return of carbon and nitrogen to the soil in trash and roots.
In many sugarcane production systems, commercial yield is measured as the fresh weight of sugarcane stems and their sucrose concentration. Hence, the water content in addition to the dry weight of the stem is simulated.
Since sugarcane is grown both as a plant and ratoon crop, the model also needs to be able to simulate differences between crop classes based on any known physiological differences between these classes.
Crop growth in the absence of nitrogen or water limitation
Thermal time
Thermal time is used in the model to drive phenological development and canopy expansion.
In APSIM-Sugarcane, thermal time is calculated using a base temperature of 9 oC, optimum temperature of 32 oC, and maximum temperature of 45 oC.
The optimum and maximum temperatures were taken from those used for maize (Jones and Kiniry, 1986).
Base temperatures for sugarcane have been variously reported between 8 oC and 15 oC (Inman-Bamber 1994b, Robertson et al, 1998).
The base of 9 oC used in APSIM sugarcane was chosen to be consistent with those studies which sampled the greatest temperature range, namely Inman-Bamber 1994b and Robertson et al (1998) who identified base temperatures of 10 oC and 8 oC respectively.
For thermal time calculations in the model, temperature is estimated every three hours from a sine function fitted to daily maximum and minimum temperatures, using the method described by Jones and Kiniry (1986).
Phenology
The sugar model uses six different stages to define crop growth and status.
| Stage | Description |
| sowing | From sowing to sprouting |
| sprouting | From sprouting to emergence |
| emergence | From emergence to the beginning of cane growth |
| begin_cane | From the beginning of cane growth to flowering |
| flowering | From flowering to the end of the crop |
| end_crop | Crop is not currently in the simulated system. |
Sprouting occurs after a lag period, set to 350 oCdays for plant crops and 100 oCdays for ratoon crops.
Provided the soil water content of the layer is adequate, shoots will elongate towards the soil surface at a rate of 0.8 mm per oCday.
The thermal duration between emergence and beginning of stalk growth is a genotype coefficient in the range 1200 to 1800 oCdays.
Although, sugarcane does produce flowers, the number of stalks producing flowers in a field is highly variable, and its physiological basis is not fully understood.
While the model structure has been developed to include flowering as a phenological stage, it has been deactivated until a better physiological basis for prediction is available.
Canopy expansion
The experimental basis for the canopy expansion model is described by Robertson et al (in press).
Briefly, green leaf area index is the product of green leaf area per stalk and the number of stalks per unit ground area.
Green leaf area per stalk is simulated by summing the fully-expanded area of successive leaves that appear on each stalk, and adding a correction factor for the area of expanding leaves (set to 1.6 leaves per stalk).
Profiles of leaf area per leaf are input as genotype coefficients.
Robertson et al (in press) found leaf appearance rates declined as a continuous function of cumulative thermal time, so that at emergence leaves took 80 oCd to appear while leaf 40 required 150 oCd.
These responses are reproduced in the model (via a series of linear interpolations) in both plant and ratoon crops.
Stalk number rises rapidly to a peak during the first 1400 oCdays from emergence, thereafter declining to reach a stable stalk number (e.g. Inman-Bamber, 1994b).
Ratoon crops commonly reach an earlier peak stalk number than plant crops, with consequently faster early canopy expansion in ratoons (Robertson et al., 1996).
In the model, the complexity of simulating the dynamics of tillering in order to predict LAI during early growth is avoided.
Instead, the crop is conceived to have a notional constant stalk number throughout growth, usually set at 10 stalks m-2 , although this value can be varied as an input.
The additional leaf area associated with tillers that appear and subsequently die, is captured via a calibrated tillering factor, that effectively increases the area of the leaves that are produced over the early tillering period.
The known faster early expansion of LAI in ratoon crops is simulated via two effects.
Firstly, the lag time for regrowth of shoots after harvest is shorter in a ratoon crop than is the equivalent thermal time for a plant crop to initiate stalk elongation.
Secondly, tillering is recognised in the model coefficients as making a larger contribution to leaf area development in a ratoon crop than a plant crop.
The daily rate of senescence of green leaf area is calculated as the maximum of four rates determined by the factors of ageing, light competition, water stress and frost.
In the model, ageing causes senescence by not allowing at any time more than 13 fully-expanded green leaves per stalk.
Light competition is simulated to induce senescence once fractional radiation interception reaches 0.85.
Water stress induces senescence once the soil water deficit factor for photosynthesis declines below 1.0.
Frosting removes 10% of the LAI per day if the minimum temperature reaches 0 oC, and 100% if it reaches -5 oC.
Root growth and development
Root biomass is produced independently from the shoot, so that a proportion of daily above-ground biomass production is added to the root system.
The proportion decreases from a maximum of 0.30 at emergence and asymptotes to 0.20 at flowering.
Root biomass is converted to root length via a specific root length of 18000 mm g-1 .
The depth of the root front in plant crops increases by 1.5 cm day-1 (Glover, 1967) from emergence, with the maximum depth of rooting set by the user.
At harvest, 17% of roots in all the occupied soil layer die (Ball-Coelho et al., 1992).
Biomass accumulation and partitioning
The sugar model partitions dry matter to five different plant pools. These are as follows:
| Plant Part | Description |
| Root | Below-ground biomass |
| Leaf | Leaf |
| Sstem | Structural component of millable stalk |
| Cabbage | Leaf sheath and tip of growing stalks etc |
| Sucrose | Sucrose content of millable stalk |
In addition to the five live biomass pools outlined above, senescent leaf and cabbage is maintained as trash on the plant or progressively detached to become residues on the soil surface.
In APSIM, the RESIDUE module (Probert et al., 1996) takes on the role of decomposition of crop residues.
LAI is used in the model to intercept incident solar radiation following Beer’s Law, using a radiation extinction coefficient of 0.38, determined by Muchow et al. (1994b) and Robertson et al. (1996).
Intercepted radiation is used to produce daily biomass production using a radiation-use efficiency (RUE) of 1.80 g MJ-1 for plant crops and 1.65 g MJ-1 for ratoon crops.
The values of RUE used in the model are those adjusted upwards from field-measured values (Muchow et al. 1994b; Robertson et al., 1996a) due to the underestimate of biomass production caused by incomplete recovery of senesced leaf material (Evenson et al., 1995; Robertson et al., 1996).
In the model, RUE is reduced if the mean daily temperature falls below 15 oC or exceeds 35 oC, and becomes zero if the mean temperature reaches 5 or 50 oC, respectively.
These effects are similar to those used in other models of C4 crop species (e.g. Hammer and Muchow, 1994).
Four above-ground biomass pools are modelled: leaf, cabbage, structural stem, stem sucrose, and an additional pool for roots that is simulated separately from above-ground production.
Between emergence and the beginning of stalk growth, above-ground biomass is partitioned between leaf and cabbage in the ratio 1.7:1 (Robertson et al., 1996a).
After the beginning of stem growth 0.7 of above-ground biomass is partitioned to the stem (Robertson et al., 1996a), with the remainder partitioned between leaf and cabbage in the ratio 1.7:1.
After a minimum amount of stem biomass has accumulated, the daily biomass partitioned to stem is divided between structural and sucrose pools, following the framework developed by Muchow et al. (1996a) and Robertson et al. (1996a). Thereafter, the stem biomass is equal to the sum of structural and sucrose pools.
If biomass partitioned to leaf is insufficient for growth of the leaf area, as determined by a maximum specific leaf area, then daily leaf area expansion is reduced.
If biomass partitioned to leaf is in excess of that required to grow the leaf area on that day, then specific leaf area is permitted to decrease to a lower limit, beyond which the “excess” biomass is partitioned to sucrose and structural stem.
A stalk growth stress factor is calculated as the most limiting of the water, nitrogen and temperature limitations on photosynthesis.
This stress factor influences both the onset and rate of assimilate partitioning to sucrose at the expense of structural stem.
Stem water content
A stem water pool is simulated for the purposes of calculating cane fresh weight and CCS%.
For every gram of structural stem grown, a weight of water is considered to have been accumulated by the cane stems.
This relationship varies with thermal time, ranging from 9 g g-1 initially, to 5 g g-1 late in the crop life cycle.
The former represents the water content of young stem (eg. cabbage) while the latter represents a combination of young stem growth and thickening of older stem.
Sucrose deposition in the stem removes water content at the rate of 1 g water g-1 sucrose.
Varietal effects
Currently varieties differ in only two respects in the model.
Firstly, Inman-Bamber (1991) found that varieties in South Africa differed in the fully-expanded area of individual leaves.
The distributions for NCo376 and N14 were taken from Inman-Bamber and Thompson (1989), while that for Q117 and Q96 was those assigned values that gave best fit to the time-course of LAI during the model calibration stage.
Secondly, Robertson et al. (1996a) found that varieties from South Africa and Australia differed in terms of partitioning of biomass to sucrose in the stem.
There is scope for incorporating other varietal differences as new knowledge becomes available.
Water deficit limitation
Soil water infiltration and redistribution, evaporation and drainage is simulated by other modules in the APSIM framework (Probert et al., 1996, Verburg et al, 1997).
Water stress in the model reduces the rate of leaf area expansion and radiation-use efficiency, via two soil water deficit factors, which vary from zero to 1.0, following the concepts embodied in the CERES models (Ritchie, 1986).
Soil water deficit factor 1 (SWDEF1), which is less sensitive to soil drying, reduces the radiation-use efficiency (i.e. net photosynthesis) and hence transpiration, below its maximum.
Soil water deficit factor 2 (SWDEF2), which is more sensitive to soil drying, reduces the rate of processes governed primarily by cell expansion, i.e. daily leaf expansion rate.
SWDEF1 and 2 are calculated as a function of the ratio of (potential soil water supply from the root system) and the (transpiration demand).
Following Sinclair (1986) and Monteith (1986), transpiration demand is modelled as a function of the (current day’s crop growth rate), divided by the transpiration-use efficiency.
When soil water supply exceeds transpiration demand, assimilate fixation is a function of radiation interception and radiation use efficiency.
When soil water supply is less than transpiration demand, assimilate fixation is a function of water supply and transpiration efficiency and the vapour pressure deficit (VPD).
Transpiration-use efficiency has not been directly measured for sugarcane, but calibration of the current model on datasets exhibiting water deficits (Robertson et al, unpubl. data) resulted in the use of a transpiration-use efficiency of 8 g kg-1 at a VPD of 1 kPa.
This efficiency declines linearly as a function of VPD (Tanner and Sinclair, 1983).
This compares with reported values of 9 g kg-1 kPa-1 for other C 4 species (Tanner and Sinclair 1983), a value that has been used in the models of sorghum (Hammer and Muchow, 1994) and maize (Muchow and Sinclair, 1991).
Potential soil water uptake is calculated using the approach first advocated by Monteith (1986) and subsequently tested for sunflower (Meinke et al., 1993) and grain sorghum (Robertson et al., 1994).
It is the sum of root water uptake from each profile layer occupied by roots.
The potential rate of extraction in a layer is calculated using a rate constant, which defines the fraction of available water able to be extracted per day.
The actual rate of water extraction is the lesser of the potential extraction rate and the transpiration demand.
If the computed potential extraction rate from the profile exceeds demand, then the extracted water is removed from the occupied layers in proportion to the values of potential root water uptake in each layer.
If the computed potential extraction from the profile is less than the demand then SWDEF2 declines in proportion, and the actual root water uptake from a layer is equal to the computed potential uptake.
In addition to the effects on canopy expansion and biomass accumulation, water stress influence biomass partitioning in the stem in two ways.
Firstly, the minimum amount of stem biomass required to initiate sucrose accumulation declines with accumulated stress.
Secondly, the daily dry weight increment between structural stem and sucrose shifts in favour of sucrose as water deficits develop.
Water excess limitation
The proportion of the root system exposed to saturated or near saturated soil water conditions is calculated and used to calculate a water logging stress factor.
This factor reduces photosynthetic activity via an effect on RUE.
Nitrogen limitation
N supply from the soil is simulated in other modules in the APSIM framework (Probert et al., 1996).
Crop nitrogen demand is simulated using an approach similar to that used in the CERES models (Godwin and Vlek 1984).
Crop N demand is calculated as the product of maximum tissue N concentration and the increment in tissue weight.
Separate N pools are described for green leaf, cabbage, millable stalk and dead leaf. The sucrose pool is assumed to have no nitrogen associated with it.
Only the leaf N concentrations influence crop growth processes. Growth is unaffected until leaf N concentrations fall below a critical concentration.
Sugarcane has been shown to exhibit luxury N uptake (Muchow and Robertson 1994 ; Catchpoole and Keating 1995) and the difference between the maximum and critical N concentrations is intended to simulate this phenomenon.
Nitrogen stress is proportional to the extent to which leaf N falls between the critical and the minimum N concentration.
Senescing leaves (and the associated leaf sheaths contained in the cabbage pool) are assumed to die at their minimum N concentrations and the balance of the N in these tissues is retranslocated to the green leaf and cabbage pools.
Maximum, critical and minimum N concentrations are all functions of thermal time, and were chosen on the basis of the findings of Catchpoole and Keating (1995) and Muchow and Robertson (1994) and subsequently refined during the model calibration.
Critical green leaf concentrations used in the model differ between photosynthetic, leaf expansion and stem growth processes.
For photosynthesis they begin at 1.2% N at emergence or ratooning and asymptote towards 0.5%N at flowering.
For leaf area expansion they are 1.3 and 0.5% N
and stem growth, 1.5 and 0.5%N.
N uptake cannot exceed N demand by the crop and is simulated to take place by mass flow in the water that is used for transpiration.
Should mass flow not meet crop demand and nitrate be available in soil layers, the approach of van Keulen and Seligman (1987) is used to simulate the uptake of nitrate over and above that which can be accounted for by mass flow.
While van Keulen and Seligman (1987) referred to this approach as “diffusion”, the routine more realistically serves as a surrogate for a number of sources of uncertainty in nitrate uptake.
Nitrogen stress also influences biomass partitioning in the stem, in a similar fashion to that described above for water stress.
Other features of the sugar module
APSIM-Sugarcane includes a number of features relevant to sugarcane production systems.
Either plant or ratoon crops can be simulated at the outset or a plant crop will regenerate as a ratoon crop if a crop cycle is being simulated.
Production systems of plant – multiple ratoon – fallow can be simulated or alternatively other APSIM crop or pasture modules can be included in rotation with sugarcane.
Trash can be burnt or retained at harvest time.
Insect or other biological or mechanical damage to the canopy can be simulated via “graze” actions.
Many sugarcane crops are “hilled-up” early in canopy development, an operation that involves the movement of soil from the interrow to the crop row.
This operation facilitates irrigation operations and improves the crop’s ability to stand upright.
APSIM-Sugarcane responds to a management event of hilling-up by removal of lower leaf area and stem from the biomass pools.
Lodging is a widespread phenomenon in high-yielding sugarcane crops.
The APSIM-MANAGER (McCown et al 1996) can initiate a lodging event in response to any aspect of the system state (eg crop size, time of year and weather).
APSIM SUGARCANE responds to lodging via four effects:
A low rate of stalk death which has been widely observed in heavily lodged crops (Muchow et al., 1995; Robertson et al., 1996; Singh et al., 2002);
A reduction in radiation use efficiency (Singh et al., 1999; Singh et al., 2002)
A reduction in the proportion of daily biomass that is partitioned as sucrose (Singh et al., 2002); and
A reduction in the maximum number of green leaves, to capture the reported reduction in leaf appearance rate and increase in leaf senescence (Singh, 2002; Singh et al., 2002).
Sugar Module Outputs
| Variable Name | Units | Description |
| Stage_name | Name of the current crop growth stage | |
| Stage | Current growth stage number | |
| Crop_status | Status of the current crop (alive, dead, out) | |
| ratoon_no | Ratoon number (0 for plant crop, 1 for 1st ratoon, 2 for 2nd ratoon, …etc) | |
| das | Days | Days after sowing (ie. crop duration) |
| Ep | mm | Crop evapotranspiration (extraction) for each soil layer |
| cep | mm | Cumulative plant evapotranspiration |
| rlv | mm.mm-3 | root length per volume of soil in each soil layer |
| esw | mm | Extractable Soil water in each soil layer |
| root_depth | mm | Root depth |
| sw_demand | mm | Daily demand for soil water |
| biomass | g.m-2 | Total crop above-ground biomass (Green + Trash) |
| green_biomass | g.m-2 | Total green crop above-ground biomass |
| biomass_n | g.m-2 | Total Nitrogen in above-ground biomass (Green + Trash) |
| green_biomass_n | g.m-2 | Amount of Nitrogen in green above-ground biomass |
| dlt_dm | g.m-2 | Daily increase in plant dry matter (photosynthesis) |
| dm_senesced | g.m-2 | Senesced dry matter in each plant pool |
| n_senesced | g.m-2 | Amount of Nitrogen in senesced material for each plant pool |
| Canefw | t.ha-1 | Fresh Cane weight |
| ccs | % | Commercial Cane Sugar |
| Cane_wt | g.m-2 | Weight of cane dry matter |
| leaf_wt | g.m-2 | Weight of plant green leaf |
| root_wt | g.m-2 | Weight of plant roots |
| sstem_wt | g.m-2 | Weight of plant structural stem |
| sucrose_wt | g.m-2 | Weight of plant sucrose |
| cabbage_wt | g.m-2 | Weight of plant cabbage |
| n_conc_cane | g.g-1 | Nitrogen concentration in cane |
| n_conc_leaf | g.g-1 | Nitrogen concentration in green leaf |
| n_conc_cabbage | g.g-1 | Nitrogen concentration in green cabbage |
| n_demand | g.m-2 | Daily demand for Nitrogen |
| cover_green | 0-1 | Fractional cover by green plant material |
| cover_tot | 0-1 | Fractional cover by total plant material (Green + Trash) |
| lai | mm-2 .mm-2 | Leaf area index of green leaves |
| tlai | mm-2 .mm-2 | Total plant leaf area index (green + senesced) |
| slai | mm-2 .mm-2 | Senesced leaf area index |
| n_leaf_crit | g.m-2 | Critical Nitrogen level for the current crop |
| n_leaf_min | g.m-2 | Minimum Nitrogen level for the current crop |
| nfact_photo | 0-1 | Nitrogen stress factor for photosynthesis |
| nfact_expan | 0-1 | Nitrogen stress factor for cell expansion |
| swdef_photo | 0-1 | Soil water stress factor for photosynthesis |
| swdef_expan | 0-1 | Soil water stress factor for cell expansion |
| swdef_phen | 0-1 | Soil water stress factor for phenology |
Sugar Model Validation Examples
Crop Growth and Development
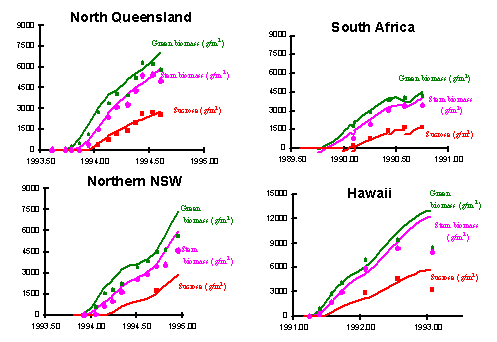
The capability of the APSIM sugar model to describe crop growth has been tested widely in a number of areas of current model application.
The following show the response of the model in simulating growth of total biomass, cane and sucrose in varying environments.
Note : Current simulations of cane water content are unreliable in some regions prior to final harvest.
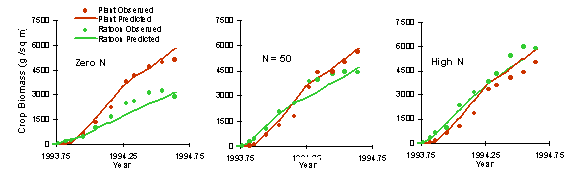
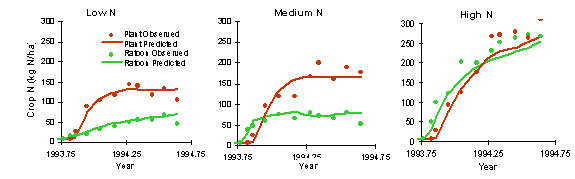
Nitrogen Uptake and Response
The applicability of the APSIM sugar model under differing nitrogen supply has been tested with several data sets.
The following example is for a plant and ratoon crop at Macknade in 1993-1994 for three nitrogen fertiliser rates (0 and 50 kg N/ha and a continuous N non-limiting treatment).
Nitrate Leaching under Sugarcane
(See Evaluation of Nitrogen fertiliser management strategies under sugarcane using APSim-Swim, Verburg et al in the Appendices)
The use of APSIM sugarcane model for nitrogen leaching study has been tested using experimental data from Bundaberg field study.
The soil was a red-yellow podsolic with a marked textual change at 0.8m depth.
Crop management included 3 nitrogen rates (0, 160 and 320 kg N/ha) for an irrigated first ratoon crop of CP51-21.
A bromide tracer was applied to some of the sub-plots.
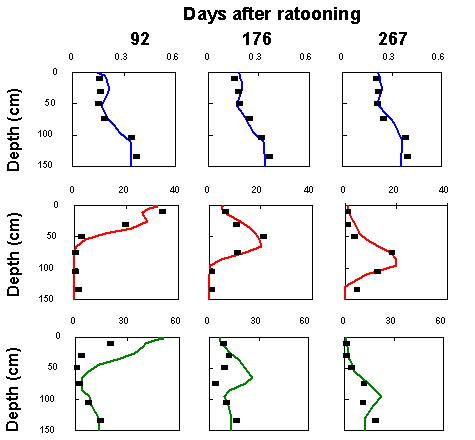
|
REFERENCES
Ball-Coelho, B. Sampaio, E. V. S. B.; Tiessen, H.; Stewart, J. W. B. (1992). Root dynamics in plant and ratoon crops of sugar cane. Plant and Soil 142:297-305.
Evenson, C. I., Muchow, R. C., El-Swaify, S. A., and Osgood, R. V. (1995). Yield accumulation in irrigated sugarcane. I. Effect of crop age and variety. (in press).
Glover, J. (1967). The simultaneous growth of sugarcane roots and tops in relation to soil and climate. Proc. S. Afr. Sugar Technol. Assoc., 143-159.
Godwin, D.C. and Vlek, P.L.G. (1984). Simulation of nitrogen dynamics in wheat cropping systems. In : Day, W. and Atkin, R.K. (eds), Wheat growth and modelling. Plenum Press, New York, pp 311-332.
Hammer, G. L. and Muchow, R. C. (1994). Assessing climatic risk to sorghum production in water-limited subtropical environments I. Development and testing of a simulation model. Field Crops Research, 36: 221-234.
Inman-Bamber NG and Thompson GD (1989). Models of dry matter accumulation by sugarcane. Proc. S. Afr. Sugar Technol. Assoc., 212-216 .
Inman-Bamber NG (1994b) Temperature and seasonal effects on canopy development and light interception of sugarcane. Field Crops Research, 36: 41-51.
Jones, C. A., Kiniry, J. R. (1986) (ors), CERES-Maize: A simulation model of maize growth and development., Texas A&M University Press, PP. 37-48.
Catchpoole, V.R. and Keating, B.A. (1995) Sugarcane yield and nitrogen uptake in relation to profiles of mineral-nitrogen in the soil. Proc. 17th Aust. Soc. Sugarcane Technol. , pp 187-192.
McCown RL, Hammer GL, Hargreaves JNG, Holzworth DP and Freebairn DM (1995). APSIM: A novel software system for model development, model testing, and simulation in agricultural systems research. Agric. Syst. 50, 255-271
Monteith, J. L. (1986). How do crops manipulate water supply and demand? Phil. Trans. Royal Soc. Lond. A , 316: 245-289.
Muchow RC, Robertson MJ, Wood AW, Spillman M F (1994a) Effect of soil fumigation on sugarcane productivity under high-yielding conditions in north Queensland.Proc. Aust. Soc. Sugar Cane Technol. , 1994 Conf., 187-92.
Muchow RC, Robertson MJ & Wood AW (1996a) Growth of sugarcane under high input conditions in tropical Australia. II. Sucrose accumulation and partitioning, and commercial yield. Field Crops Research 48:27-36.
Muchow, R. C. and Robertson, M. J. (1994). Relating crop nitrogen uptake to sugarcane yield. Proc.Aust. Soc. Sugarcane Technol., 1994, 122 – 130.
Muchow RC, Robertson MJ,Wood AW & Keating BA (1996b) Effect of nitrogen on the time-course of sucrose accumulation in. Field Crops Research 47: 143-153.
Muchow RC, Spillman MF, Wood AW & Thomas MR (1994b) Radiation interception and biomass accumulation in a sugarcane crop grown under irrigated tropical conditions. Aust. J. Agric. Research 45, 37-49.
Muchow, R. C., Wood, A. W. and Robertson, M. J. (1995) Does stalk death set the yield ceiling in high yielding sugarcane crops? Proc. Aust. Soc. Sugar Cane Technol. 17, 142 – 148.
Muchow RC, Wood AW, Spillman MF, Robertson M J & Thomas MR (1993) Field techniques to quantify the yield-determining processes in sugarcane. I. Methodology. Proc. Aust. Soc. Sugar Cane Technol. , 1993 Conf., 336-343.
Probert ME, Dimes JP, Keating BA, Dalal RC, Strong WM (1997). APSIM’s water and nitrogen modules and simulation of the dynamics of water and nitrogen in fallow systems. Agric. Syst. (in press).
Ritchie, J. T. (1986). In: Jones, C. A., Kiniry, J. R. (1986) (ors), CERES-Maize: A simulation model of maize growth and development., Texas A&M University Press, PP. 37-48.
Robertson MJ, Muchow RC, Inman-Bamber, NG, Wood AW (1996) Relationship between biomass and sucrose accumulation in sugarcane. in “Sugarcane: Research Towards Efficient and Sustainable Production” Wilson JR, Hogarth DM, Campbell JA and Garside AL (eds). CSIRO Division of Tropical Crops and Pastures, Brisbane. 1996, pp 84-86.
Robertson, M.J. Bonnett, G.D. and Campbell. J.A. (1998).Temperature and leaf area expansion of sugarcane: integration of controlled-environment, field and model studies. Australian Journal of Plant Physiology. 25, 819-828.
Robertson, M. J., Wood, A. W., and Muchow, R. C. (1996) Growth of sugarcane under high input conditions in tropical Australia. I. Radiation use, biomass accumulation and partitioning. Field Crops Res . 48, 11 – 25.
Sinclair, T. R. (1986). Water and nitrogen limitations in soybean grain production. I Model development. Field Crops Research , 15: 125-141.
Singh, G. (2002) Constraints to High Yield and CCS in Large and Lodged Cane Crops. Ph D Thesis (submitted to James Cook University).
Singh, G., Chapman, S. C., Jackson, P. A. and Lawn, R. J. (1999) Yield accumulation in sugarcane under wet tropical conditions – effect of lodging and crop age. Proc. Aust. Soc. Sugar Cane Technol. 21, 241 – 245.
Singh, G., Chapman, S. C., Jackson, P. A. and Lawn, R. J. (2002) Lodging reduces sucrose accumulation of sugarcane in the wet and dry tropics. Aust. J. Agric. Res. 53, 1183 – 1195.
Tanner, C. B. and Sinclair, T. R. (1983). Efficient water use in crop production: research or re-search? in “Limitations to Efficient Water use in Crop Production” (Eds. H. M. Taylor, W. R. Jordan, T. R. Sinclair). pp.1-27. ASA, Madison, Wisconsin.
Van Keulen, H. and Seligman, N.G. (1987). Simulation of water use, nitrogen nutrition and growth of a spring wheat crop. Pudoc Wageningen, 310pp.
Verburg, K., Ross, P.J. and Bristow, K.L. (1997) SWIMv2 Manual. CSIRO Division of Soils Divisional Report, CSIRO, Australia, 93 pp. (in press).
